SpaceX launched its 10,000th Starlink satellite on October 19th at 17.39 UTC. Some observers said there had been a malfunction but SpaceX later confirmed all the satellites had been deployed and the valuable…
Blog
-
Cellular Switch Found To Drive Lung Scarring in Mice
Pulmonary fibrosis is a deadly disease in which the lungs become thickened and scarred, gradually losing their ability to deliver oxygen to the body. Now, scientists at UC San Francisco have identified a key cellular switch that drives this…
Continue Reading
-
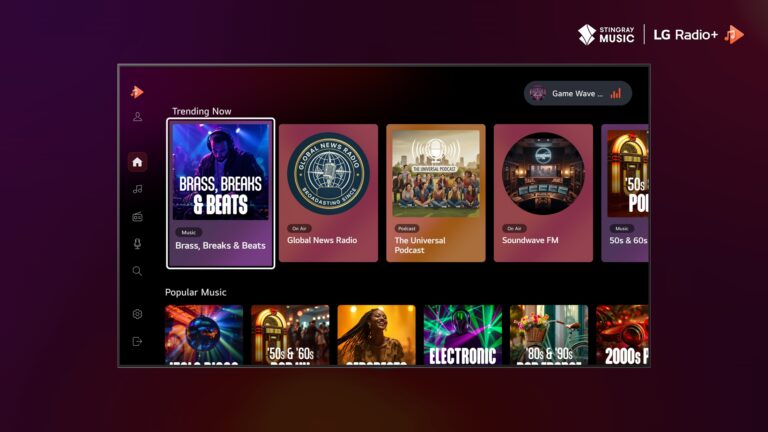
LG Radio+ adds Stingray Music
LG Electronics has partnered with Stingray Music to upgrade its free ad-supported audio streaming service, LG Radio+. Available on LG Smart TVs running webOS 6.0 and above in the UK, the US, France, Germany,…
Continue Reading
-

Airbus Cathay SAF co-investment SAF partnership
Hong Kong, China, 21 October 2025 – Airbus and the Cathay Group have announced a joint investment of up to US$70 million to accelerate the development of sustainable aviation fuel (SAF) production in Asia and globally.
The agreement was announced in Hong Kong on the sidelines of the IATA World Sustainability Symposium at a ceremony hosted by Cathay Chief Operations and Service Delivery Officer Alex McGowan and Airbus President Asia-Pacific Anand Stanley.
Under the terms of the partnership, the two companies will collaborate to identify, evaluate and invest in projects that support the scaling of SAF production towards 2030 and beyond. Projects will be assessed based on their commercial viability, technology maturity, and potential for long-term offtake.
Scaling SAF requires deep collaboration across the value chain, including from policymakers and investors to SAF producers and customers. This co-investment agreement reflects the spirit of partnership with Airbus and Cathay teaming up to accelerate production capability for more meaningful impact.
“SAF remains the most important lever for Cathay and the wider aviation industry to drive toward our decarbonisation goals,” said Alex McGowan, Chief Operations & Service Delivery Officer, Cathay. “This co-investment partnership with Airbus underscores our commitment to building a stronger, more scalable SAF industry. It complements our broader strategy of investing in the technologies and production capacity needed for the future, including our recent investment in the oneworld BEV SAF Fund. Meanwhile we are expanding SAF usage today through partnerships with like-minded organisations.”
“This agreement reflects the shared commitment of Airbus and Cathay to make a real difference,” said Anand Stanley, President Asia Pacific, Airbus. “The production and distribution of affordable SAF at scale requires an unprecedented cross-sectoral approach. Our partnership with Cathay is a concrete example of how we catalyse production in the most suitable locations to serve our customers.”
The joint commitment also includes collaboration to advocate for supportive SAF policies on both the supply and demand side across Asia. With the region’s strong potential in feedstock supply, production capacity, and its vibrant aviation market, Airbus and Cathay aim to leverage their global experience to help shape policies that make SAF more accessible and affordable in this part of the world.
Airbus and Cathay have a long-standing partnership dating back to 1989, when the airline signed its first order for Airbus aircraft. Today, the Cathay Group operates 86 Airbus aircraft with over 70 more on order for future delivery.
@Cathay @Airbus #SAF
Continue Reading
-

Breakthrough eye implant restores reading vision in blind patients
image: ©Vertigo3d | iStock A new eye implant paired with augmented reality glasses has allowed patients with previously untreatable blindness to read again
The results of a European clinical trial, published in The New…
Continue Reading
-

7 Workplace Trends That Will Define 2026
As technology reshapes workplaces and careers, the way we work is set to undergo profound change as we move into the second half of the decade. For those wanting to adapt and stay ahead of the curve, these changes can be anticipated by tracking…
Continue Reading
-

Scientists Discover Building Blocks Of Life In Ice Around Forming Star In Neighboring Galaxy
Astronomers have found complex organic ice outside the Milky Way for the first time. The discovery shows that the building blocks of life could arise early in the universe – and under a variety of conditions, said Leiden astronomer Will…
Continue Reading

FLASH: The Yuan 4 billion anode materials project settles in Zhejiang Province
According to the latest news from the Zhoushan High-tech Industrial Park on the morning of October 18, Liyang Tianmu Pioneer Battery Material Technology Co., Ltd. officially signed an agreement with Zhejiang Zhoushan High-tech Industrial Park to…
Continue Reading

Role of Faricimab In Refractory Neovascular Age-Related Macular Degene
Introduction
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss among individuals aged 50 years and older worldwide.1 The global prevalence of AMD was estimated at approximately 196 million in 2020, with projections rising to 288 million by 2040 due to increasing life expectancy and aging populations in both developed and developing regions.1,2 In India, the burden of AMD is similarly rising: recent population-based studies have reported an overall prevalence of any AMD of 1.4–2.7%, with neovascular AMD (nAMD) accounting for a substantial proportion of vision-threatening disease.3 As life expectancies increase and lifestyles change, the number of individuals at risk for AMD in India is expected to grow significantly over the next decade, underscoring the need for effective management strategies.
Since the introduction of intravitreal anti-VEGF therapy in the mid-2000s, the management of nAMD has been revolutionized. Pegaptanib was the first approved agent, but ranibizumab (Accentrix®, Novartis India), bevacizumab, and aflibercept (Eylea®, Regeneron) showed superior efficacy in pivotal trials.4–7 Ranibizumab improved or maintained vision in over 90% of eyes at one year (MARINA, ANCHOR),4,5 while aflibercept demonstrated non-inferiority with fewer injections (VIEW 1, 2).6 More recently, brolucizumab (Beovu®, Novartis)7 offered longer dosing intervals, though safety concerns, particularly intraocular inflammation (IOI), have limited its uptake.7,8 Despite these advances, a significant subset of eyes exhibits persistent fluid or recurrent exudation, indicating “refractory” nAMD.9,10 Such cases often require monthly injections with suboptimal anatomical and functional outcomes, highlighting the need for therapies that target additional pathways involved in disease pathogenesis.
One such emerging option is faricimab (Vabysmo®, Roche/Genentech, Basel, Switzerland), a bispecific monoclonal antibody that simultaneously inhibits VEGF-A and angiopoietin-2 (Ang-2).11 By dual targeting, faricimab aims not only to suppress angiogenesis but also to stabilize the retinal vasculature and reduce inflammation and vascular leakage mediated by Ang-2/Tie-2 dysregulation.11 In the Phase III TENAYA and LUCERNE trials, faricimab administered every 8- or 16-weeks achieved visual and anatomical outcomes non-inferior to aflibercept dosed every 8 weeks, with a median durability of 12 weeks in treatment-naïve nAMD eyes.12 These results suggested that dual pathway inhibition could potentially improve durability and efficacy over monotherapy. Importantly, faricimab’s distinct mechanism offers a theoretical advantage in eyes that have demonstrated suboptimal response to conventional anti-VEGF-A monotherapy.
Real-world studies of faricimab are emerging, but data specifically in refractory nAMD eyes remain limited.13–15 A retrospective series in the United States reported that a subset of refractory nAMD eyes switched to faricimab after inadequate response to aflibercept achieved decreased central retinal thickness and stability in vision over twelve months, suggesting potential benefits in a real-world context.13 A study from Japan reported that while 40% of aflibercept-resistant eyes could be extended to a bimonthly regimen after switching to faricimab, 59.2% ultimately discontinued the therapy for various reasons.14 To date, no published data have described the use of faricimab in refractory nAMD patients from India, where treatment access, patient demographics, and disease characteristics may differ from Western populations. This lack of local evidence creates a knowledge gap, as socioeconomic factors, genetic predispositions, and treatment adherence patterns can influence outcomes in the Indian setting.15,16
nAMD represents a growing public health challenge, particularly in aging populations such as India’s. While anti-VEGF agents have revolutionized the management of nAMD, a subset of eyes remains refractory to standard therapies, leading to ongoing vision loss and treatment burden. Faricimab’s dual inhibition of VEGF-A and Ang-2 offers a promising therapeutic alternative in these eyes. Given the limited real-world evidence in refractory eyes and the absence of data from India, there is a clear need to evaluate faricimab’s effectiveness in this subgroup. Our study was therefore designed to assess the anatomical and functional outcomes of faricimab in eyes with refractory nAMD in a real-world Indian cohort.
Materials and Methods
This retrospective, multicenter investigation included patients managed between January 2024 and December 2025 at two tertiary care centers in India: B B Eye Foundation, Kolkata, India and Shantilal Shanghvi Eye Institute, Mumbai, India. The protocol received ethical clearance from both institutions’ review boards (BB Eye Foundation Ethics Committee and Shantilal Shanghvi Foundation Ethics Committee). All procedures adhered to the tenets of the Declaration of Helsinki, and written informed consent was obtained from each participant for treatment and data usage.
Eligible eyes were those diagnosed with nAMD that had demonstrated a refractory response to prior anti-VEGF therapy; specifically, eyes that had received at least three consecutive monthly injections of aflibercept or brolucizumab yet continued to exhibit persistent intraretinal fluid (IRF) and/or subretinal fluid (SRF) on spectral-domain OCT. Patients were required to be 50 years or older, have a confirmed diagnosis of nAMD in the study eye, and have completed a minimum of six months of follow-up at one of the two participating centers after switching to faricimab.
Eyes were excluded if any concurrent retinal or choroidal pathology could confound the diagnosis or treatment response; for example, macular neovascularization (MNV) secondary to high myopia, inflammatory causes, and other. Additional exclusions included significant media opacities (such as dense cataract or vitreous hemorrhage) that precluded reliable OCT imaging, a history of intraocular surgery (other than uncomplicated cataract extraction) within the preceding three months, concurrent diabetic retinopathy requiring treatment, advanced glaucoma, or any other ocular condition that, in the investigator’s judgment, would interfere with outcome assessment or patient safety.
At enrollment, each patient underwent a comprehensive ophthalmic evaluation performed by fellowship-trained retina specialists. Best-corrected visual acuity (BCVA) was recorded using a Snellen chart and converted to logarithm of the minimum angle of resolution (logMAR) for analysis. Intraocular pressure was measured by Goldmann applanation tonometry. Anterior segment examination was carried out with slit-lamp biomicroscopy, and dilated fundus evaluation employed 90D and 20D lenses. SD-OCT (Cirrus HD-6000; Carl Zeiss Meditec, Dublin, CA, USA) captured macular volume scans (6×6 mm, 512×128 scans) to quantify IRF, SRF, and pigment epithelial detachment (PED).
Faricimab (6.0 mg/0.05 mL) was administered on a pro re nata (PRN) basis. Injections were performed under sterile conditions in a designated minor procedure room. After topical anesthesia (proparacaine), 5% povidone-iodine was applied to the ocular surface and periocular area. The pars plana was entered with a 30-gauge needle 3.5 mm posterior to the limbus in phakic eyes (4.0 mm in pseudophakic eyes). No routine prophylactic topical antibiotics were prescribed.
Patients were evaluated monthly for the first three months post-injection and then at physician discretion, based on disease activity. At each visit, BCVA, intraocular pressure, slit-lamp biomicroscopy, and dilated fundus examination were repeated. SD-OCT scans were acquired at every follow-up to document changes in IRF, SRF, and PED. Any unscheduled visits prompted additional assessments if patients reported new symptoms (eg, diminished vision, pain, photopsia).
The primary efficacy endpoint was the change in BCVA from baseline to the final follow-up visit. Secondary endpoints included changes in the central foveal thickness, the proportion of eyes showing complete resolution of IRF, SRF, and PED on SD-OCT. Imaging at each center was evaluated by a single experienced grader each (RB and JS). In the event of any uncertainty or discrepancy in interpretation, findings were jointly reviewed between them, and a final consensus was reached to ensure consistency in assessment.
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA). Continuous variables, such as BCVA and CFT, were expressed as mean ± standard deviation (SD). Changes from baseline at each follow-up visit were evaluated using paired t-tests, with significance set at P<0.05.
For categorical variables including IRF, SRF, PED, and any fluid, McNemar’s test was employed to compare paired proportions at baseline and at 6 months. A two-sided P<0.05 was considered statistically significant.
Results
A total of 24 eyes from 24 patients with refractory nAMD were included in this study. The mean age of patients was 68.1 (± 10.6) years. Prior to switching to faricimab, the eyes had received an average of 11.4 (± 9.1) anti-VEGF injections, primarily aflibercept or brolucizumab. Over the six-month study period, eyes received a mean of 2.63 ± 1.34 faricimab injections (range, 1–5) on a pro-re-nata (PRN) basis: 25.0% (n=6) of eyes received one injection, 29.2% (n=7) two injections, 16.7% (n=4) three injections, 16.7% (n=4) four injections, and 12.5% (n=3) five injections. Table 1 demonstrates the demographic characteristics and treatment profile of the study eyes.
|
Table 1 Demographic Characteristics and Treatment-Profile of the Study Population
|
Best-Corrected Visual Acuity Outcomes
The mean BCVA at baseline was 0.66 (± 0.4) logMAR. Statistically significant improvements in BCVA were noted at all follow-up time points. At 1 month, mean BCVA improved to 0.47 (± 0.34) logMAR (mean change: −0.19 [± 0.26]; P=0.0003). At 2 months, further improvement was observed (0.35 [± 0.32]; mean change: −0.3 [± 0.3]; P<0.0001), with continued gains at 3 and 6 months (0.27 [± 0.26] and 0.27 [± 0.27], respectively; mean change from baseline: −0.38 [± 0.31]; P<0.0001 for both) (Table 2).
 |
Table 2 Changes in the Best-Corrected Visual Acuity (BCVA) and Central Foveal Thickness (CFT) in the Study Population
|
Anatomical Outcomes
In terms of anatomical response, the mean CFT at baseline was 471.1 (± 246.4) µm. CFT showed a statistically significant reduction at each follow-up visit: 337.3 ± 198.3 µm at 1 month (mean change −133.8 [± 133.9] µm; P<0.0001), 265.1 ± 90.7 µm at 2 months (−206.0 [± 184.2] µm; P<0.0001), 217.7 ± [41.3] µm at 3 months (−253.4 [± 205.6] µm; P<0.0001), and 209.4 [± 36] µm at 6 months (−261.7 [± 208.3] µm; P<0.0001) (Table 2).
At baseline, SRF was present in 22 of 24 eyes (91.66%), IRF in 16 eyes (66.67%), and PED in 11 eyes (45.83%). Over the six-month follow-up period, significant anatomical improvements were noted across most parameters. By month 6, SRF had completely resolved in 20 of the 22 affected eyes (90.9%), with only 2 eyes showing persistent SRF. Importantly, no new cases of SRF developed during follow-up. Similarly, IRF resolved in 14 of the 16 eyes (87.5%) in which it was initially present. The remaining two eyes exhibited persistent IRF, and no new cases were noted in the previously unaffected cohort. PED demonstrated a comparatively modest response. Of the 11 eyes with PED at baseline, 6 eyes (54.5%) showed complete resolution, while 5 eyes continued to exhibit persistent PED at the end of 6 months. There were no instances of new PED development in eyes that were initially PED-free. Using McNemar’s test for paired binary outcomes, the reduction in both SRF and IRF was found to be statistically significant (P <0.001 and P=0.0006, respectively), while the change in PED did not reach statistical significance (P=0.32). Complete resolution of fluid was noted in 20/24 eyes (83.33%) at the end of six-months, which was statistically significant (P=0.00002). Table 3 demonstrates the changes in the fluid and PED status of the study eyes.
 |
Table 3 Proportion of Eyes with Resolution of Fluid and Pigment Epithelial Detachment (PED)
|
Safety Analysis
No ocular or systemic adverse events were reported during the study period.
Discussion
In this retrospective real-world analysis of 24 eyes with treatment-refractory nAMD, switching to faricimab on a PRN regimen was associated with meaningful functional and anatomical improvements over six months. Visual acuity gains were both early and sustained, with mean BCVA improving from 0.66 logMAR at baseline to 0.27 logMAR at six months. Central retinal thickness decreased steadily, accompanied by high rates of fluid resolution: over 90% of eyes with baseline SRF and nearly 88% of eyes with IRF achieved complete resolution, while PED showed more modest improvement. Overall, 83% of eyes were fluid-free at six months, and no unexpected safety issues were observed. These findings suggest that faricimab may provide anatomical stability and functional benefit in patients with chronic, previously treated nAMD under real-world conditions.
The current study adds to mounting evidence that faricimab can meaningfully improve outcomes in eyes with nAMD that have proven refractory to prior anti-VEGF therapy. In our real-world cohort of refractory nAMD eyes, conversion to faricimab was associated with significant anatomical improvements; notably reductions in retinal thickness, fluid, and PED, while visual acuity was generally maintained. These findings are consistent with previous reports, including those by Tamiya R et al,17 who observed significant anatomical improvements along with preservation of visual acuity in patients with anti-VEGF resistant nAMD. Similarly, Bantounou et al18 reported favorable anatomical outcomes and stable visual acuity, achieved with a reduced number of injections. Together, these data suggest that faricimab can resolve persistent edema that has failed to clear with other agents, even when short‐term functional gains are modest. The current study’s outcomes thus align with the emerging consensus that faricimab may rescue patients in whom prior anti-VEGF therapy has plateaued, reducing fluid burden without compromising safety.
Mechanistically, faricimab’s efficacy in this setting is readily explained by its unique dual-target action. Faricimab is a bispecific monoclonal antibody that simultaneously binds vascular endothelial growth factor A (VEGF-A) and angiopoietin-2 (Ang-2).11,12 The VEGF pathway is the well-known driver of neovascular growth and leakage in AMD, and all prior first-line treatments (bevacizumab, ranibizumab, aflibercept, brolucizumab) target VEGF‐A or its family. Angiopoietins (primarily Ang-1 and Ang-2) regulate vascular stability via the Tie2 receptor: Ang-1/Tie2 signaling promotes quiescence and tight endothelial junctions, whereas elevated Ang-2 (usually released from hypoxic or stressed endothelium) competes with Ang-1 and effectively destabilizes vessels, making them more permeable and prone to inflammation.19 In nAMD, chronic hypoxia and inflammation drive Ang-2 upregulation, so that even if VEGF is neutralized, ongoing Ang-2–mediated permeability and inflammatory signaling can sustain fluid. By simultaneously inhibiting Ang-2 and VEGF-A, faricimab promotes vascular stabilization and mitigates inflammatory processes.17–19 In practical application, this results in a more comprehensive inhibition of both angiogenic signaling and vascular permeability pathways. Thus, faricimab’s mechanism directly addresses a hypothesized contributor to refractory fluid: elevated Ang-2 and persistent vascular leak despite prior VEGF blockade. If a patient’s persistent edema is partly driven by Ang-2–mediated inflammation and microvascular instability, faricimab is the first available therapy that can counteract both pathogenic arms simultaneously.
Prior strategies for refractory AMD, including lateral switches among VEGF agents or the use of higher-dose or longer-acting molecules, have had variable and often incomplete success.20,21 While newer agents like brolucizumab showed potent drying effects and extended durability in pivotal trials, concerns over intraocular inflammation (IOI) and rare but severe instances of retinal vasculitis have limited their adoption in clinical practice.8,9 Faricimab, by targeting an additional angiogenic pathway without a marked increase in inflammatory risk, offers an appealing alternative.17–19 We hypothesize that by targeting a complementary angiogenic pathway, one not addressed by earlier agents, faricimab underlies the superior visual and anatomical outcomes we observed, all while avoiding the immune-mediated toxicity profile characteristic of brolucizumab.
Beyond its dual-target action, faricimab offers practical advantages that are especially relevant in a high-burden setting. The pivotal TENAYA and LUCERNE trials showed that faricimab dosed up to every 16 weeks achieved non-inferior visual outcomes compared to aflibercept every 8 weeks.12 By two years, ~60–80% of patients on faricimab could be extended to 12- or 16-week intervals.12 This durability was mirrored in DME trials (YOSEMITE/RHINE) and small real-world studies;22 for example, Penha et al23 report that faricimab treated patients often achieved 12-week or longer dosing schedules in practice. In our study, over six months, more than half of eyes (54.2%) required two or fewer injections after switching; 25.0% received a single injection and 29.2% received two, underscoring the potential to reduce treatment burden. In India, where adherence is often compromised by travel difficulties, cost, and comorbidities, such extended intervals can be transformative. Frequent anti-VEGF visits (4–8 week intervals) impose heavy logistic and financial strain. Indeed, even in well-resourced settings only a small minority of patients can sustain ≥12-week intervals with standard care.18 By contrast, faricimab’s protocol (with the option of Q12–16W dosing) directly addresses an unmet need in real-world management of recalcitrant nAMD, potentially improving adherence and outcomes over time.
It should be noted that global experience with faricimab in refractory AMD is still emerging. A few recent reports illustrate its promise but also highlight the need for more data, especially in diverse populations. Baek et al24 found that faricimab reduced injection burden and improved visual and anatomical outcomes in eyes unresponsive to other agents. Bantounou et al18 observed that faricimab produced rapid fluid resolution and decreased injection frequency in previously treated nAMD, again with stable VA. Tamiya et al17 observed that over half of their aflibercept-refractory eyes had fluid reduction after one faricimab injection, and 25% achieved a dry macula at 2 months without recurrence for up to 4 months. These series consistently report anatomical gains with visual stabilization or improvement. However, none of these studies included substantial numbers of Indian patients. Our study is thus timely: by providing real-world data on faricimab in refractory nAMD in an Indian context, it fills a critical gap. To our knowledge, no prior published series from India has evaluated faricimab in this specific population. Given potential racial, genetic and healthcare differences, it cannot be assumed that Western findings extrapolate perfectly to Indian eyes. Our study’s population, often older patients with significant macular pathology, limited resources, and irregular follow-up, reflects “real life” conditions in India. The fact that faricimab produced clear anatomic benefits in this cohort supports its generalizability and suggests it is a viable tool in the Indian retina armamentarium.
Nonetheless, the current study has inherent limitations. As a retrospective, single-arm review, it cannot prove efficacy with the rigor of a randomized trial. There is no concurrent control group, and selection bias (which eyes were chosen for switching) likely influenced outcomes. Follow-up is relatively short, and end-points like VA are affected by ceiling/floor effects and chronic scarring in these eyes. We also did not analyze patient-reported outcomes or long-term retreatment rates. On the other hand, the study’s strengths include its multi-center design and “real-world” heterogeneity; we included patients who in practice would not meet strict trial criteria (eg very chronic lesions, multiple previous injections). The findings therefore complement the controlled trials by showing what happens in everyday clinics. Importantly, no unexpected safety issues arose: faricimab was well-tolerated, with no cases of IOI being reported.
Conclusion
In summary, the current study shows that faricimab, by neutralizing both VEGF-A and Ang-2, delivers meaningful visual and anatomical gains in refractory nAMD while addressing dual angiogenic pathways. Importantly, over half of eyes required two or fewer injections over six months, underscoring a substantial reduction in treatment burden. In an Indian context, where real-world data are limited, these findings suggest that retina specialists can expect outcomes on par with global reports. Practically, faricimab may be indicated in cases with persistent edema or suboptimal response to other anti-VEGF therapies. Although vigilance for IOI remains essential, the balance of robust efficacy and fewer injections makes faricimab a valuable switch option. However, these findings should be interpreted with caution. Larger, prospective studies with longer follow-up are needed to validate and refine retreatment strategies in this cohort.
Disclosure
J.US is affiliated with Shantilal Shanghvi Foundation (SSF), outside the submitted work. The authors declare that they have no other competing interests in this work.
References
1. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e116. doi:10.1016/S2214-109X(13)70145-1
2. Sheth JU, Stewart MW, Narayanan R, et al. Macular neovascularization. Surv Ophthalmol. 2025;70(4):653–675. doi:10.1016/j.survophthal.2024.08.003
3. Hamati J, Prashanthi S, Narayanan R, et al. Prevalence of age-related macular degeneration and associated factors in Indian cohort in a tertiary care setting. Indian J Ophthalmol. 2023;71(10):3361–3366. doi:10.4103/IJO.IJO_199_23
4. Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65.e5. doi:10.1016/j.ophtha.2008.10.018
5. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMoa054481
6. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration [published correction appears in Ophthalmology. Ophthalmology. 2012;119(12):2537–2548. doi:10.1016/j.ophtha.2012.09.006
7. Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi:10.1016/j.ophtha.2019.04.017
8. Chakraborty D, Maiti A, Sheth JU, et al. Brolucizumab in neovascular age-related macular degeneration – indian real-world experience: the BRAILLE study – fifty-two-week outcomes. Clin Ophthalmol. 2022;16:4303–4313. doi:10.2147/OPTH.S395577
9. Chakraborty D, Maiti A, Sheth JU, et al. Brolucizumab in neovascular age-related macular degeneration – indian real-world experience: the BRAILLE study. Clin Ophthalmol. 2021;15:3787–3795. doi:10.2147/OPTH.S328160
10. Ashraf M, Banaee T, Silva FQ, Singh RP. Switching anti-vascular endothelial growth factors in refractory neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2018;49(3):166–170. doi:10.3928/23258160-20180221-03
11. Agostini H, Abreu F, Baumal CR, et al. Faricimab for neovascular age-related macular degeneration and diabetic macular edema: from preclinical studies to phase 3 outcomes. Graefes Arch Clin Exp Ophthalmol. 2024;262(11):3437–3451. doi:10.1007/s00417-024-06531-9
12. Khanani AM, Kotecha A, Chang A, et al. TENAYA and LUCERNE: two-year results from the phase 3 neovascular age-related macular degeneration trials of faricimab with treat-and-extend dosing in year 2. Ophthalmology. 2024;131(8):914–926. doi:10.1016/j.ophtha.2024.02.014
13. Rush RB. One-year outcomes of faricimab treatment for aflibercept-resistant neovascular age-related macular degeneration. Clin Ophthalmol. 2023;17:2201–2208. doi:10.2147/OPTH.S424315
14. Kataoka K, Itagaki K, Hashiya N, et al. Six-month outcomes of switching from aflibercept to faricimab in refractory cases of neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2024;262(1):43–51. doi:10.1007/s00417-023-06222-x
15. Chakraborty D, Das S, Maiti A, et al. Clinical evaluation of faricimab in real-world diabetic macular edema in India- a multicenter observational study. Clin Ophthalmol. 2025;19:269–277. doi:10.2147/OPTH.S502033
16. Soman M, Nair I, Sheth JU, Nair U. Innovator Versus Biosimilar Ranibizumab in Polypoidal Choroidal Vasculopathy: real-World Evidence. Ophthalmol Ther. 2022;11(3):1175–1186. doi:10.1007/s40123-022-00507-w
17. Tamiya R, Hata M, Tanaka A, et al. Therapeutic effects of faricimab on aflibercept-refractory age-related macular degeneration. Sci Rep. 2023;13(1):21128. doi:10.1038/s41598-023-48190-6
18. Bantounou MA, Elsheikh M, Ijasan A, Santiago C. Real-world experience of intravitreal faricimab injection in previously treated neovascular age-related macular degeneration eyes: a case series. BMC Ophthalmol. 2025;25(1):117. doi:10.1186/s12886-025-03953-9
19. Khanani AM, Russell MW, Aziz AA, et al. Angiopoietins as potential targets in management of retinal disease. Clin Ophthalmol. 2021;15:3747–3755. doi:10.2147/OPTH.S231801
20. Yiu G, Gulati S, Higgins V, et al. Factors Involved in Anti-VEGF treatment decisions for neovascular age-related macular degeneration: insights from real-world clinical practice. Clin Ophthalmol. 2024;18:1679–1690. doi:10.2147/OPTH.S461846
21. Fu Y, Zhang Z, Webster KA, Paulus YM. Treatment strategies for anti-VEGF resistance in neovascular age-related macular degeneration by targeting arteriolar choroidal neovascularization. Biomolecules. 2024;14(3):252. doi:10.3390/biom14030252
22. Wong TY, Haskova Z, Asik K, et al. Faricimab treat-and-extend for diabetic macular edema: two-year results from the randomized phase 3 YOSEMITE and RHINE trials. Ophthalmology. 2024;131(6):708–723. doi:10.1016/j.ophtha.2023.12.026
23. Penha FM, Masud M, Khanani ZA, et al. Review of real-world evidence of dual inhibition of VEGF-A and ANG-2 with faricimab in NAMD and DME. Int J Retina Vitreous. 2024;10(1):5. doi:10.1186/s40942-024-00525-9
24. Baek SC, Jeong A, Min Sagong M. Real-world efficacy of faricimab in patients with treatment-resistant neovascular age-related macular degeneration: outcomes at six months. J Retina. 2024;9:150–155. doi:10.21561/jor.2024.9.2.150
Continue Reading
Just a moment…
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
